|
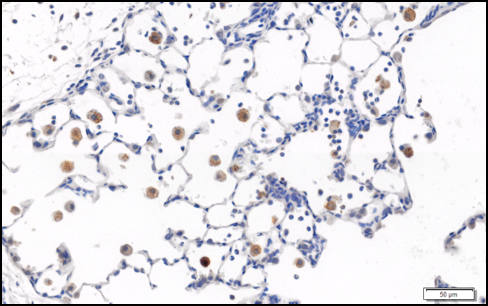
Well, it’s over. Weeks and weeks of pouring over stained slides, slaving over the VS120, perfecting the poster and my paper, and it’s done. It has definitely been an amazing amazing experience. I practically ended up living in the lab and I did not like waking up at 7 am every morning, but I would not exchange the experience for anything. Plus I loved it so much that I’m now staying there! Until school starts. Besides being an actual part of actual science, I can take so many other valuable lessons from this experience. First off, I’d just like to say that the food at the symposium was so amazing, I’m still not over it. I need to eat that pasta again. But besides the food, the whole thing was awesome. It was great to hear about everyone’s projects (even though some of the comp sci/engineering ones went completely over my head) and to see what they’ve worked on. Plus, it was just an awesome atmosphere, especially during the poster presentations, where everyone was so professional and I felt like I was a real scientist, instead of some random high schooler. And they gave us free stuff! Which I was not expecting and was awesome. And we got to see a laser show. And of course, we got our stipends. I have yet to deposit my check, though. But yes, my last week and “last day” was quite an experience. I definitely encourage you guys to apply. Research is not for everyone, and this also gives you a good opportunity to look critically at this field and decide if it’s going to be in your future. For me, I basically came in with the thought that oh, I’ll get my MD, and then maybe do some research on the side after I’m established. Now, coming out of this program, I’m sincerely considering a PhD. And there is no doubt in my mind that even if I decide to forward with an MD that I will be doing research as early and as often as possible. This experience also left me with lab based skills that you just can’t get in school. Things that scientists take for granted but are such important parts of research. I’m not talking about all the procedures, like IHC and protein assays and Western blots and cell counting and all that. But the simple stuff like pipetting, changing alcohols, taking biohazard trash to be autoclaved, things like that. And reading papers! I can assure you that you do not know how time-consuming and mentally exhausting it is to go through droves and droves of literature. It also left me with an amazing network of individuals in the scientific community. Luckily for me, the PI I worked under is practically the queen of toxicology in the science world, and she knows everyone. And she probably knows at least one person in every university in the US, so if I am ever in need of a lab to work in, she’ll put in a good word. Plus, it left me with a pretty awesome letter of recommendation. And another good part about this summer - this is actual research that I can submit to science fairs which might actually win some prizes (maybe)! I am so excited to enter these fairs and see if what we did holds up to other projects. I honestly think it’s a really legitimate piece of science and is interesting to boot, so it should be able to hold its own. Finally, LSC PIS has also left me with an awesome network of students that I’ll always have a connection to, and who will always help each other out. I’m like 75% sure we’ll see at least one of each other again at a college campus (and we’ll hear Jason’s name on the news because now he found the cure for cancer or something, I swear, that kid… go check out his Kickstarter, Polyfuge, the affordable microcentrifuge here). But enough about how amazing and spectacular and unique this experience was (oh, and one final note, this experience gives you what you put in. I put in the work, I paid attention, I wasn’t careless, and so I got to reap the benefits. There are others that did not, for whom in the end, this experience did not pay off much.) Let’s talk about my actual research. So after switching back and forth between projects for a bit, we ended up settling on the effects of nitrogen mustard as a whole, with a special focus on preliminary treatment results with gadolinium chloride. Here’s my abstract: Nitrogen mustard (NM) is a cytotoxic alkylating agent known to cause acute lung injury which progresses to fibrosis. Following NM exposure there is a sequential accumulation of pro-inflammatory/cytotoxic M1 and anti-inflammatory/wound repair M2 macrophages in the lung which have been implicated in disease pathogenesis. Gadolinium chloride (GdCl3) is a rare earth metal which has been shown to suppress M1 macrophage activation. In these studies, we analyzed the effects of GdCl3 on macrophages accumulating in the lung in response to NM as a potential treatment to counteract the effects of NM. GdCl3 (7 mg/kg) was administered i.v. to male Wistar rats 24 hr prior to i.t. instillation of PBS or NM (0.125 mg/kg). Lung tissue was collected 3 d and 7 d later. Increased numbers of iNOS+ and COX-2+ pro-inflammatory M1 macrophages were observed in the lung 3 days following administration of NM and NM + GdCl3. At 7 days, however, there are greater numbers of CD163+ anti-inflammatory M2 macrophages in NM + GdCl3 treated rats, relative to NM treated rats, but decreased expression of heme oxygenase-1 (HO-1), a marker for oxidative stress. These findings suggest that GdCl3 modulates the response of macrophages to NM, resulting in increases in anti-inflammatory macrophages and reduced oxidative stress. These findings may be useful in the development of therapeutics aimed at mitigating NM-induced lung inflammation and injury. That probably all sounds like mumbo jumbo, so let me break it down a little further. (If you don’t want to read about my research, skip to the end for tips for applying to LSC PIS.) Nitrogen mustard (NM) is closely related (structurally) to mustard gas, which I’m sure you guys have heard of, the chemical warfare agent that was most recently used in Syria in 2016. Well, NM is less volatile and therefore safer to work with, so we use that to study mustard gas instead. So what we did (or had to have someone do, because we’re under 18 and not certified) is inject mice with either NM or PBS (a control saline solution). Each of these groups was also divided into two, one that received the treatment gadolinium chloride (GdCl3) and one that received a vehicle, or PBS placebo. These were rats that were taken down (aka euthanized) and whose lungs we used for our tissue specimens. When looking at the lung, we were looking for inflammation. One of the characteristics of inflammation is the accumulation of macrophages, a type of white blood cells. There are two types: M1 and M2. M1 is pro-inflammatory (so it’s seen as the “bad” macrophage), while M2 is anti-inflammatory (“good”). (If you’re more curious about this, google “Laskin macrophage Star Wars” - my PI wrote an awesome paper comparing the two.) Anyway, we used IHC, or immunohistochemistry, to stain for these different macrophages. The ones that expressed whatever antibody we used (ie had that marker on their membrane) would stain a shade of brown. (For example, this pic shows brown macrophages in the NM 3d HO-1 IHC.) We used more than just the three mentioned in the abstract, but those are the most important. iNOS and COX-2 are both markers for M1 macrophages, so in that case, more brown = bad (for the animal, at least). CD163 is an M2 marker, so brown = good. (Again, this is literally the largest generalization in the world, you need both types of macrophages for a proper injury response, but for this purpose, we’ll call it good and bad.) HO-1 is also mentioned in the abstract, and that’s just a general marker for oxidative stress, aka injury.
We primarily looked for the presence of these macrophages (in order to do that, we stained each section of tissue with a different antibody corresponding to different markers) at both 3 days and 7 days past exposure for each group. In total, we looked at 5 different antibodies, and each antibody was used on 8 different tissue groups, so 40 IHC images in all. But let me explain our results a little, at least the M1-M2 parts. So, what we found was that immediately following injury, at 3 days, there was an increase in M1 macrophages, a pro-inflammatory response, in the NM group compared to the controls. Over time, at 7 days, there was an increase in M2 anti-inflammatory response in NM vs controls. Basically what this means is that acutely, there is more M1 activity, and chronically, more M2 activity. And I could talk about the implications of this forever, but let’s move on. Looking at the groups treated with gadolinium, there appears to be a decrease in M1 at both 3 and 7 days, and an increase in M2 at 3 and 7 days. What this is telling us is that gadolinium is both suppressing M1 macrophage activity and upregulating M2 activity. Which, to sum it all up, means it’s working, at least on this base level observing just images. (I say this because we don’t actually know the cell counts, so we don’t know if it’s significant enough to say that it is a viable treatment, and regardless, tons more research needs to be done before anything can even be whispered.) Of course, we had a lot of other supporting data points, as all research must have, like our protein assays and PAS staining and histopathological changes (although these mainly focused on just the effects of NM). If we had more time we would include actual cell counts - like literally the number of brown macrophages per IHC tissue specimen. (In fact, this is what I’m doing at the lab right now and oh my god it is the most tedious thing in the world.) And we would also include flow cytometry data - a process that fluorescently tags cells that have certain markers so you can see what types of cells were in the sample. (We did do flow a few weeks ago, but it was for another experiment.) Anyway, that’s pretty much my entire paper (or at least, ⅔ of it) in 5ish paragraphs. I honestly didn’t intend to talk that much about it and if you’re still reading then wow, I commend your perseverance. That was probably a crap explanation and if you’re genuinely curious about learning more, please reach out to me, I can talk about this stuff for weeks, literally. Anyway, that was my final product of this summer (and man, the poster ended up looking absolutely amazing and professional and oh so glossy too). And all of that is actually only about a half of the work I did this summer, the rest was dedicated towards random projects of other people in the lab. But to finish up this final update, here’s just some tips/things to keep in mind when applying to LSC that I found applicable. (Also, definitely let me know if you’re applying, I’d love to help and/or divulge more details.) 1. Pay attention to your cover letter and resume. It’s one of the larger parts of your application, so make sure they are written well. (For my guide on how to write a killer resume, click here.) For your cover letter, google what it generally should have (it should be a sort of summary for your interest, skills/qualifications/experience, enthusiasm, stuff like that) and keep it to maybe 3 good length paragraphs. 2. Pick your fields of research interest carefully. Yes, I know you are super passionate about medicine and health, but so are 200 other kids applying. There are only so many medicine/health mentors out there, and definitely not enough for the 200, maybe not even enough for 15. So pick a different field that you have a strong interest in for your first choice (you can still keep medicine/health for your second choice) - but make sure you are passionate/well-versed on it and are willing to research in that field. Don’t pick ecological biology if you’re not willing to go out in the field and collect seaweed, or whatever they do. (Also, this pretty much only applies to those that are thinking of medicine/health as their number 1, because literally, I mean literally everyone chooses that. If you’re genuinely into physics or something, go for it. So much math though. I could never do it.) 3. Be realistic in where you’re willing to travel, but try to branch out a little. One of the biggest factors for acceptance is where you live/are willing to commute to where the actual research is going to take place. If there is only 1 mentor in the South Jersey area, there will only be 1, max 2 students in that area (or who listed that area as one they are willing to commute to) that will get in, even if 5 people deserved that spot. Most of their mentors are either from Rutgers Piscataway/New Brunswick or universities in the Jersey City area. (And they’re tied up with mentors in Princeton and Columbia too - only engineering ones, sadly.) So those are where you see most LSC students from. For example, if you are the only person that said they are willing to travel to Hoboken and there’s a mentor at Stevens, they’re not going to waste that mentor by not matching you up with him (unless you really suck as an applicant). *Word of Caution* This does not mean list every NJ or NY county (yes, there are New York - mostly NYC - positions available). This will not help you. Be realistic and honest. If you are a strong enough applicant, you can still get into the more densely applicant populated areas. 4. Don’t stress too much about the essays. Make sure you write well and passionately and don’t have egregious mistakes, but it’s not the end of the world if your essay doesn’t stand out. Use that time to prepare for the interview instead. 5. Man oh man, prepare for that interview. Know yourself, your strengths, your weaknesses, how to describe yourself in a sentence or 30 seconds and all that jazz. This is what killed me the first time I applied. It was my first ever interview for anything, I was not confident, I had not prepared enough, plus everyone else in my interview group was a year older than me and considerably more accomplished than I was then. Don’t write off the interview as, “I’m a people person, I got this.” It is a real interview where they will ask you strange introspective questions as well as random general rapid fire science questions, nothing is off the table. Plus it’s a heck of an interview, it’s like 3 hours or something. But go in confident, prepared to talk about yourself and your experience with science. That’s all for this one guys, my last LSC update. I was planning on writing a more in-depth general LSC PIS article, covering more than just tips, but I don’t know if I will. Do you want me to? If so, let me know, and tell me any questions you have. But for now, thanks for reading!
6 Comments
Vish
1/1/2022 06:18:31 pm
I recently learned about this program(I'm a sophomore), and needless to say, I definitely want to apply. The opportunity to do research over the summer is almost too good to pass up. I'm actually really surprised at the lack of information available about this program; it seems like you're the only person whose talking about it!
Reply
Sara
3/28/2022 08:27:02 pm
Hi, Vish!
Reply
Vish
3/28/2022 09:08:01 pm
Hey Sara!
Reply
Sweksha
4/13/2022 08:16:11 pm
Hi Sara! I just saw this, I also applied to the LSC program this year and I got the email a couple days ago that they were getting ready to let people know next steps in the next two weeks. Hope we both get in!
Reply
Sara
3/28/2022 09:41:26 pm
Thank you so much! Best of luck on whatever you're doing as well (:
Reply
Sar_bha
5/1/2022 10:46:35 pm
Hi everyone, I am a sophomore and have received an invite for an interview next Saturday. Anyone else in the same group?
Reply
Your comment will be posted after it is approved.
Leave a Reply. |
Home |
Blog Archive |
My Old Blog |